Applying one of the oldest natural forces known to science, magnetism, researchers at the University of Illinois have developed an imaging technology that noninvasively moves nanoparticles inside the body in order to target tumor cells and other tissue with a high degree of specificity. The contrast agent technology enables micron scale tissue detection and manipulation and represents, the researchers write, “a new methodology for contrast enhancement and therapeutic interventions in molecular imaging.”
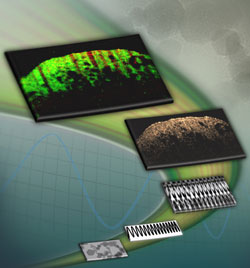
By taking advantage of the magnetic properties of iron-oxide nanoparticles, researchers were able to demonstrate for the first time in vivo magnetomotive optical coherence tomography (MMOCT) imaging of targeted magnetic nanoparticles (MNPs) in a preclinical mammary tumor model. The development of these contrast agents for imaging is an important step in biomedical research because the technology demonstrated site-specific molecular imaging that isn’t possible with other contrast agents.
The study’s results were published in a paper, In vivo magnetomotive optical molecular imaging using targeted magnetic nanoprobes, that appeared online in the Proceedings of the National Academy of Sciences April 19. Corresponding author is Beckman Institute researcher Stephen Boppart, M.D., Ph.D.
“What is novel about this whole approach and this work is that we’re using external magnetic forces to move nanoparticles within tissue, to modulate them,” Boppart said. “Most other particles will localize somewhere and typically sit there. They may provide a signal change, but they don’t physically move. No other particle is dynamic like this, which is a unique way to generate contrast.”
The paper reports on the method’s use in imaging mammary tumor tissue but Boppart said that the technology has many potential applications.
“I believe this represents an entirely new class of imaging agents that we can use to tell more about the tissue, for diagnostic purposes and for therapeutic techniques,” Boppart said.
The magnetic nanoparticles can be used with magnetic resonance imaging (MRI) as well as OCT and, potentially, other imaging technologies, enabling biomechanical tissue measurements, contrast, and therapy through hyperthermia.
Boppart said the technique uses magnetic forces to move nanoparticles bound to proteins, cells, tissue; phase-sensitive OCT then measures changes in light scattering from the movement. The method’s uniqueness comes from the ability to target the nanoparticles to a specific site, such as a tumor, where their multifunctionality can then be utilized.
The nanoparticles can provide information on contrast (especially valuable when it comes, for example, to distinguishing between healthy cells and cancerous cells) due to the fact that the MNPs are magnetic while the rest of the tissue is not. Another use is to record biomechanical tissue measurements (such as cell elasticity and viscosity, important factors in disease detection). It works by using a magnetic field to make the nanoparticles vibrate, thereby providing signals from the tissue through the rate and frequency of the movements.
Another use enabled by the technology is therapeutic. By turning up the frequency of the magnetic field, the nanoparticles move so quickly they begin to heat up, providing a possible treatment tool.
“So now we have a platform where we can target these to a tumor, find them with contrast, measure the mechanical properties, and treat it right there,” Boppart said.
The magnetic nanoparticles in this project were antibody functionalized to target the human epidermal growth factor receptor 2 (HER2 neu) protein, a receptor which is over expressed in breast cancer. The fabrication of the antibody-directed functionalized nanoparticles was also rather unique for biomedicine: conjugating the antibody to the nanoparticle in a specific way to provide the highest target specificity to the receptor.
To read the paper, click here.
