Article
 Yi "Edwin" Sun
Yi "Edwin" SunYi “Edwin” Sun, a Ph.D. candidate in electrical and computer engineering at the University of Illinois Urbana-Champaign and member of the Beckman Institute’s Biophotonics Imaging Laboratory headed by Stephen Boppart, explored how deep learning methods can make polarization-sensitive optical coherence tomography, or PS-OCT, more cost-effective and better equipped to diagnose cancer in biological tissues.
The paper, titled “Synthetic polarization-sensitive optical coherence tomography by deep learning,” was published in npj Digital Medicine.
OCT systems are common clinically and are used to generate high-resolution cross-sectional images of regions in the human body. Sun and his team developed a groundbreaking method of applying software to the OCT tool to provide polarization-sensitive capabilities — without the cost and complexity that accompany hardware-based PS-OCT imaging systems.
“We’re trying to replace the hardware associated with PS-OCT,” Sun said. “However, [it] is still in the stage of development and research. By adding a deep learning model on top of an OCT system, suddenly we arrive at PS-OCT capabilities without the traditional added hardware.”
OCT is a non-invasive imaging test that uses light waves to determine the properties of a biological sample. However, by enabling the tool to use polarization sensitivity, scientists can detect relevant information that OCT cannot capture on its own. For instance: OCT can differentiate tissue in a manner that’s precise and when larger features are clear; PS-OCT can detect abnormalities on a deeper level, differentiating microstructural features such as collagen fiber orientations that change in a cancer-infected area compared to a normal area.
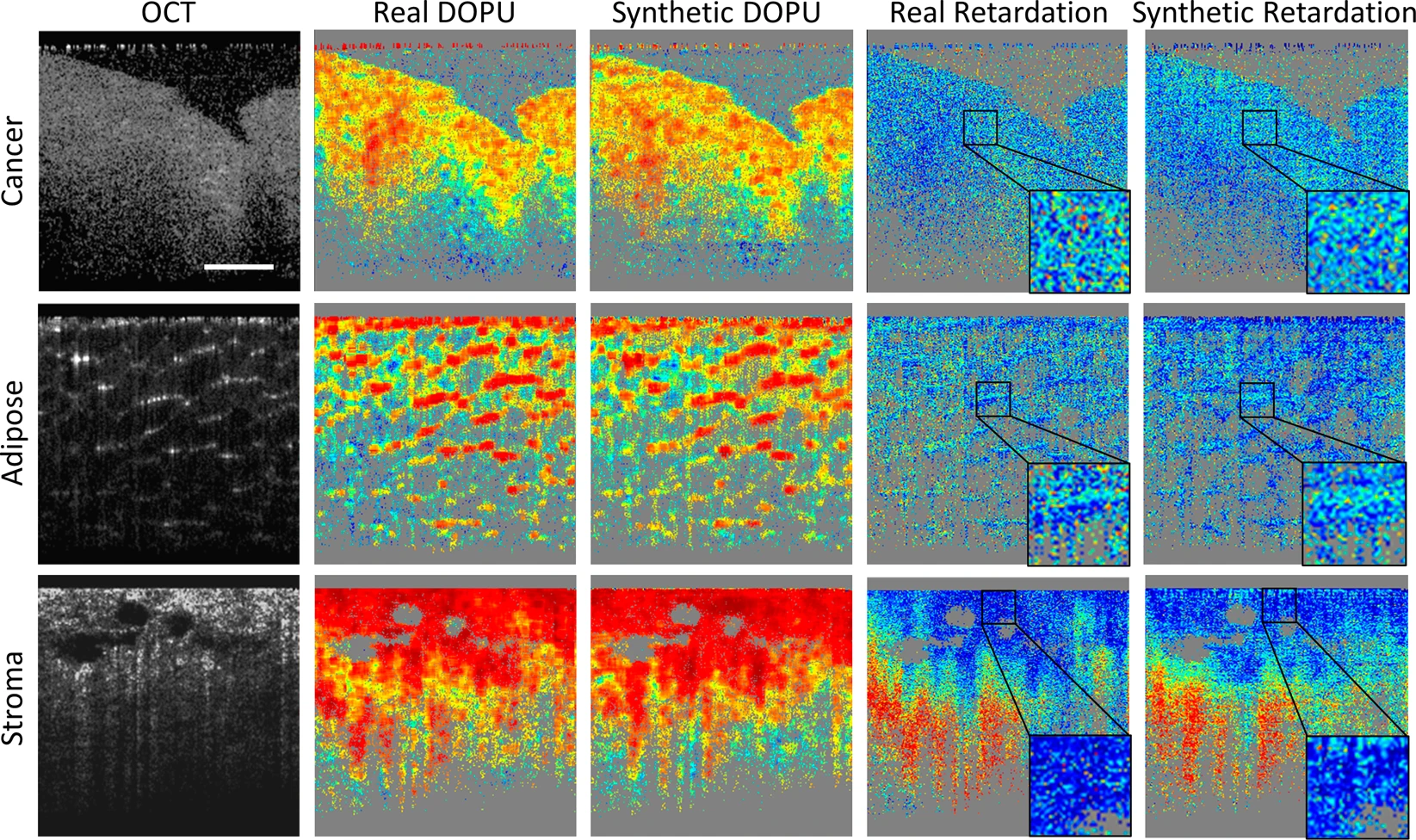 Representative computational PS-OCT images from cancer, adipose, and stroma tissue specimens, compared with the real PS-OCT images. Source: npj Digital Medicine
Representative computational PS-OCT images from cancer, adipose, and stroma tissue specimens, compared with the real PS-OCT images. Source: npj Digital Medicine“We proved that applying our method to other systems can generate a PS-OCT contrast, and that this model can be used on many OCT systems to help us differentiate cancer tissues and other types of tissues much better than OCT systems alone,” Sun said. “This is a huge improvement, making this system better for cancer diagnoses.”
Deep learning, a subset of machine learning, enabled Sun’s team to create software that can pair with OCT systems to deliver polarization sensitivity.
“Deep learning enabled a more advanced method of picking up subtle features in images, which can be used for more accurate segmentation and classification. It also allows the imaging tool to use multiple layers to pick up spatial features in an image,” Sun said.
By applying historical data, the deep learning methods assist with accurate diagnoses and even medical predictions. Sun's team tested their model by predicting what a photo of a lush summer forest might look like in December: barren, grey, perhaps a smattering of ice and snow in the trees. With this concept in mind, the images from OCT systems, coupled with this deep learning approach, can even predict the PS-OCT images that would have come from the more complex and costly PS-OCT systems.
 Steve BoppartGiven that Sun and his team’s research was a pioneer study, it may take a few years, and an abundance of data, for synthetic PS-OCT to reach the clinical stages. Once it does, clinicians may be able to apply this model to commercial systems and have a greater ability to detect cancer, using the PS-OCT generated images to assist in cancer diagnosis.
Steve BoppartGiven that Sun and his team’s research was a pioneer study, it may take a few years, and an abundance of data, for synthetic PS-OCT to reach the clinical stages. Once it does, clinicians may be able to apply this model to commercial systems and have a greater ability to detect cancer, using the PS-OCT generated images to assist in cancer diagnosis.
“Edwin’s study truly highlights the power and potential of AI and deep learning approaches to predict and generate synthetic PS-OCT images from standard OCT images, a type of image-to-image translation. With the increasing use of OCT across medical fields, this advance will likely have a broad impact, and ultimately help to improve the detection and diagnosis of disease,” said Boppart, Sun’s Ph.D. thesis advisor who is both a physician and UIUC professor of engineering.
This research was supported in part by grants from the National Cancer Institute and the National Institutes of Health.
Editor’s note: the paper associated with this research can be found here: https://www.nature.com/articles/s41746-021-00475-8
Beckman Institute for Advanced Science and Technology