Article
Opaque structures and bulk tissues such as whole animals are difficult to visualize because they scatter light strongly, creating poor contrast images. A new technique developed at the Beckman Institute is one step closer to overcoming those hurdles.
The Quantitative Light Imaging Laboratory is interested in improving the imaging of such tissues. The lab’s latest paper, “Epi-illumination Gradient Light Interference Microscopy for Imaging Opaque Structures,” looks at one such method. The paper was published in Nature Communications.
The researchers have developed the epi-GLIM technique, which can be used to image label-free images with high resolution.
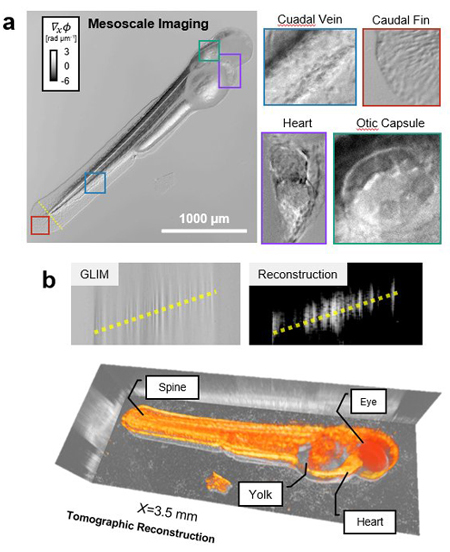
“We use white light which consists of many different colors. Using these multiple beams of light allows us to create a contrast because different portions of the tissue delays the light differently and, together, the waves reveal the structure one thin optical slice at a time. Because of the reflection geometry, this technique allows us to look at completely opaque samples,” said Gabriel Popescu, a professor of electrical and computer engineering and the head of the Quantitative Light Imaging Laboratory.
The visualization technique is based on interferometry, an investigative technique that uses the interaction of different light beams. “We upgraded a conventional microscope by adding a modulator in the light path,” said Mikhail Kandel, a doctoral student in electrical and computer engineering and a member of Popescu’s lab. “This lets us control the contrast of the image and the interference patterns.”
The researchers also have modeled theoretically the phenomenon of backscattering. “In common imaging, some of the scattered light goes in the forward direction. But some of it comes back the other side after interacting with the sample,” Kandel said.
The epi-GLIM instrument can be used to visualize diverse bodies. “We can use it to inspect semiconductors, high-density microplates that can be used for medicine screening, and animals like zebrafish,” Kandel said.
“This module can be attached to existing microscopes,” Popescu said. “We are currently collaborating with Martha Gillette’s and Catherine Best’s lab on neuroscience applications.”
“This is an exciting development in quantitative phase imaging. In principle this technique, combined with cell type specific labeling, could be used to map the entire cellular composition and wiring of an entire mouse brain,” Popescu said.
The paper, “Epi-illumination Gradient Light Interference Microscopy for Imaging Opaque Structures,” can be found online: DOI: 10.1038/s41467-019-12634-3.
Beckman Institute for Advanced Science and Technology