Article
Trillions of periodical cicadas — several species of the genus Magicicada that emerge every 13 or 17 years — broke soil across the Eastern U.S. this summer. News outlets likened the event to Armageddon, an apocalypse or an invasion. But what about using words like mesmerizing? Mysterious? Magical?
The genus name Magicicada refers to the sheer magnitude of cicadas synchronously crawling up through the earth to reach sunlight. This year is a special one for the state of Illinois: both the 13-year cicadas of the Great Southern Brood XIX and the 17-year cicadas of the Great Northern Brood XIII emerged in massive numbers. This simultaneous emergence has not occurred since 1803 and will not occur again for another 221 years.
It was prime time for entomologists and researchers at the Beckman Institute for Advanced Science and Technology at the University of Illinois Urbana-Champaign.
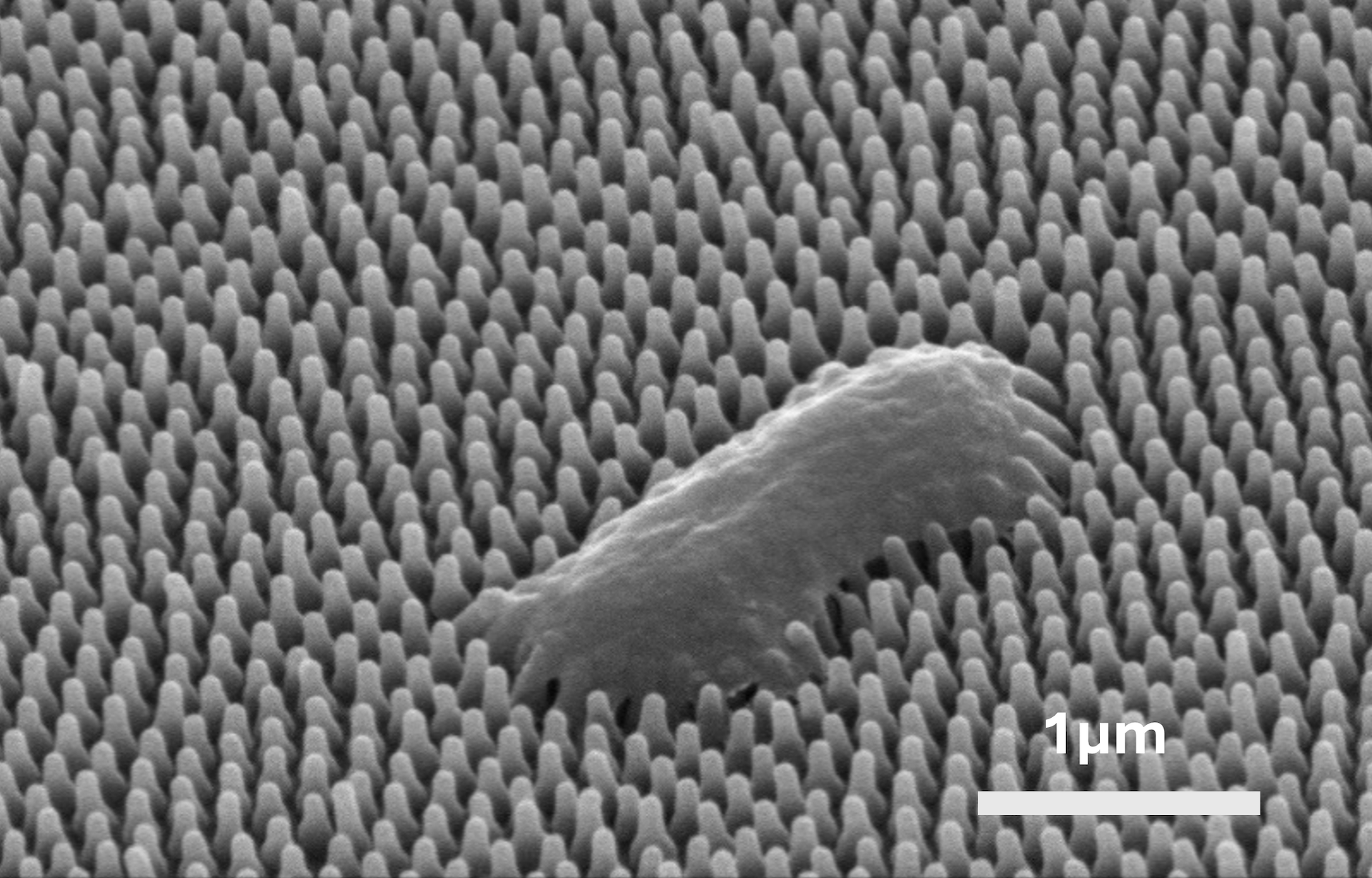 Scanning electron microscope image of a Pseudomonas aeruginosa bacterium, destroyed by nanopillars, on a cicada wing. Scale bar is 1 micrometer. Credit: Yutao Chen.
“We use cicadas and other insects as inspiration for engineering new materials. As biologists, we also want to be able to use these materials to then go in the opposite design direction. So, the prototypes that we created, and which may
result in marketable new surfaces for various industries, can also be used to help us explain fundamental biological questions about natural selection,” said Marianne Alleyne, a professor of entomology and mechanical science and engineering at Illinois.
Scanning electron microscope image of a Pseudomonas aeruginosa bacterium, destroyed by nanopillars, on a cicada wing. Scale bar is 1 micrometer. Credit: Yutao Chen.
“We use cicadas and other insects as inspiration for engineering new materials. As biologists, we also want to be able to use these materials to then go in the opposite design direction. So, the prototypes that we created, and which may
result in marketable new surfaces for various industries, can also be used to help us explain fundamental biological questions about natural selection,” said Marianne Alleyne, a professor of entomology and mechanical science and engineering at Illinois.
Her lab’s work revolves around bioinspired design: the process of learning from nature to develop new materials and technologies.
 Yutao Chen at the Beckman Institute. Credit: Elizabeth Bello, Beckman Communications Office.
Yutao Chen, a biologist and graduate student in Alleyne’s
ABC Lab, is studying the antibacterial properties of cicada wings to fabricate functional cicada-inspired surfaces.
Yutao Chen at the Beckman Institute. Credit: Elizabeth Bello, Beckman Communications Office.
Yutao Chen, a biologist and graduate student in Alleyne’s
ABC Lab, is studying the antibacterial properties of cicada wings to fabricate functional cicada-inspired surfaces.
“Cicada wings are superhydrophobic, meaning they’re really waterproof, and they also have excellent antibacterial properties,” Chen said.
What is the secret behind these cicada wing superpowers?
To the naked eye, the translucent wings appear smooth and featureless. Operating the environmental scanning electron microscope in Beckman’s Microscopy Suite, Chen magnifies a cicada wing 10,000 times. Zooming in, swirling patterns emerge, and microscopic features called nanopillars come into focus.
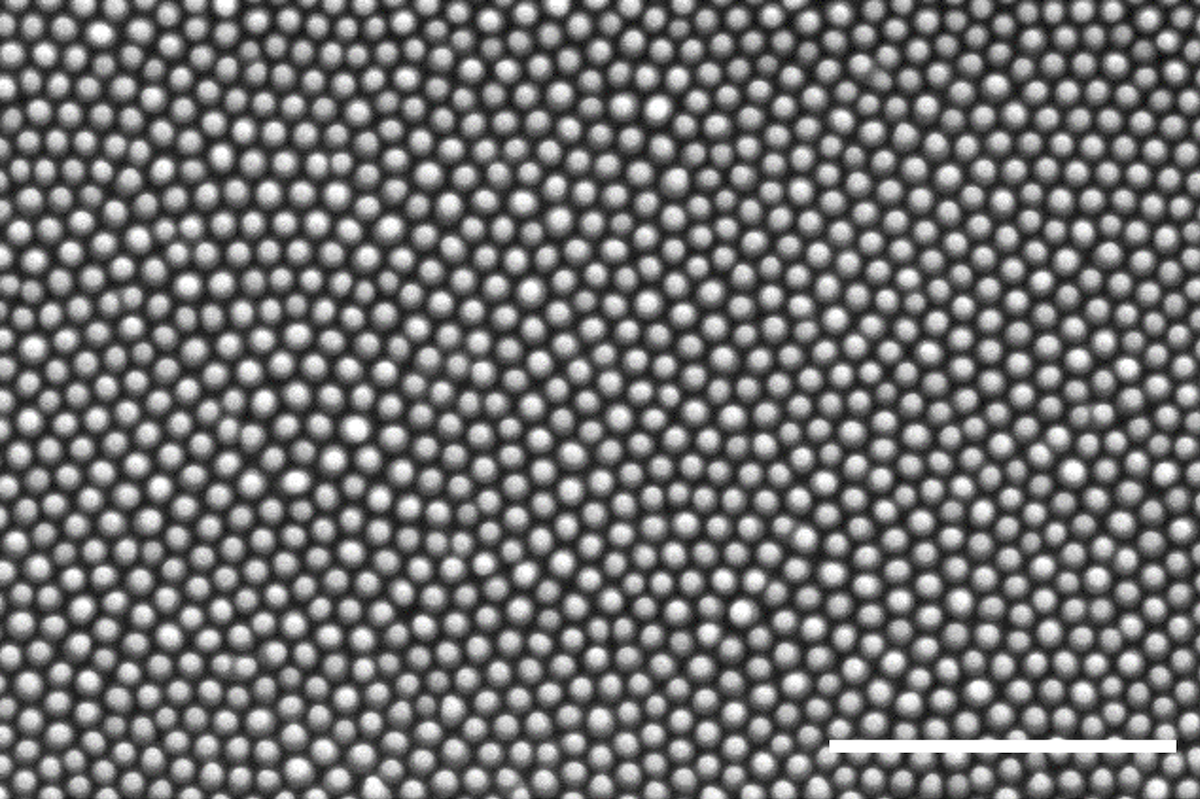 Scanning electron microscope image of cicada wing nanopillars. Scale bar is 2 micrometers. Credit: Yutao Chen.
Each nanopillar is approximately 150 nanometers wide and 200 to 400 nanometers tall. In comparison, a human hair is about 1000 times thicker than a single nanopillar. The nanopillars are distributed uniformly across each wing but can vary in size
depending on the species. They create a rough surface, giving the wings their hydrophobic, or water-repellant, and antibacterial functionalities.
Scanning electron microscope image of cicada wing nanopillars. Scale bar is 2 micrometers. Credit: Yutao Chen.
Each nanopillar is approximately 150 nanometers wide and 200 to 400 nanometers tall. In comparison, a human hair is about 1000 times thicker than a single nanopillar. The nanopillars are distributed uniformly across each wing but can vary in size
depending on the species. They create a rough surface, giving the wings their hydrophobic, or water-repellant, and antibacterial functionalities.
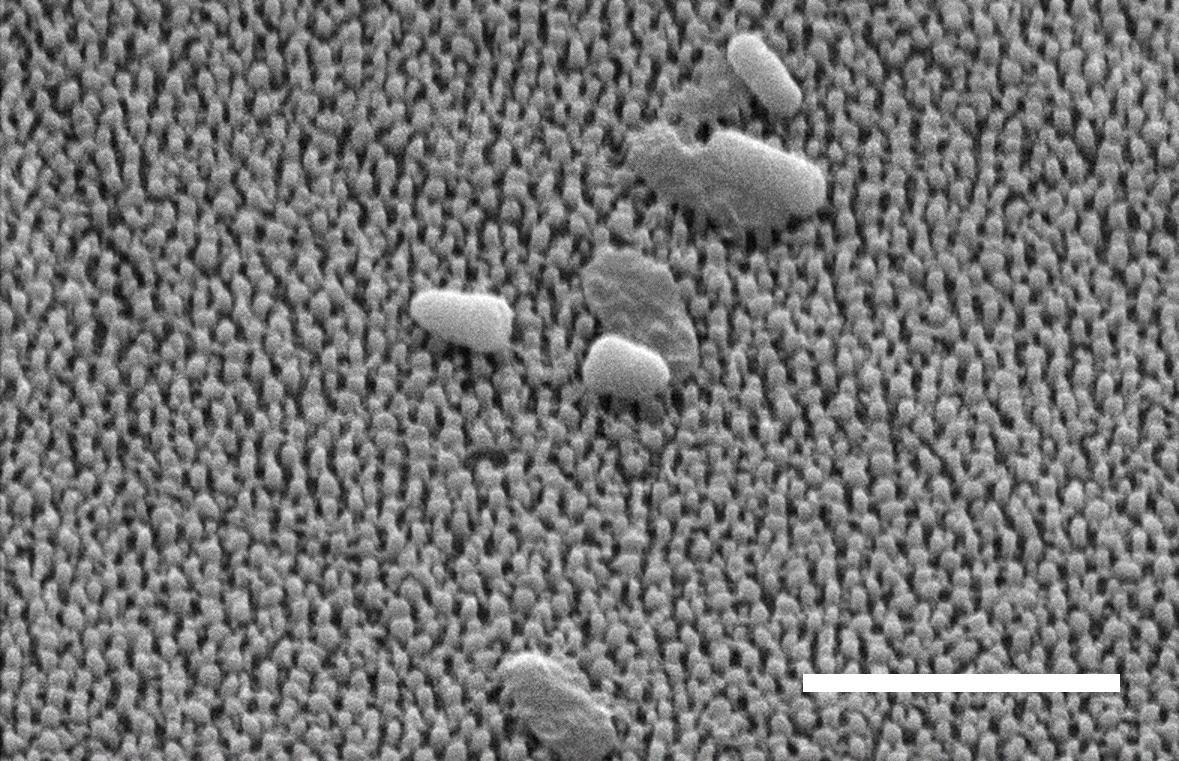 Scanning electron image of Chen's replicated polystyrene surface with punctured P. aeruginosa bacteria cells and intact bacteria cells. Scale bar is 3 micrometers. Credit: Yutao Chen.
When microbes land or move on the nanopillars, their outer membrane becomes damaged. Microbial contamination threatens cicadas and is a prevalent issue in human society: in shipping industries, underwater pipelines, medical implants and other
devices and appliances, Chen said.
Scanning electron image of Chen's replicated polystyrene surface with punctured P. aeruginosa bacteria cells and intact bacteria cells. Scale bar is 3 micrometers. Credit: Yutao Chen.
When microbes land or move on the nanopillars, their outer membrane becomes damaged. Microbial contamination threatens cicadas and is a prevalent issue in human society: in shipping industries, underwater pipelines, medical implants and other
devices and appliances, Chen said.
Efforts to deter microbes from materials are usually in the form of surface coatings which become damaged and lose efficacy over time. Antibiotics are commonly used to treat bacteria during an infection, but overuse eventually leads to microbial resistance.
“It’s imperative to develop durable surfaces that are mechanically antibacterial,” Chen said.
The nanoscale protrusions on cicada wings are the perfect inspiration to develop these new materials.
Chen uses a flexible and versatile nanoscale replication method called nanoimprinting lithography to mimic the nanoscopic features of cicada wings. The replicates are made of polystyrene, a type of polymer material that is not inherently antibacterial. Once textured with the right sized nanopillars, the polystyrene becomes bactericidal, or able to destroy bacteria.
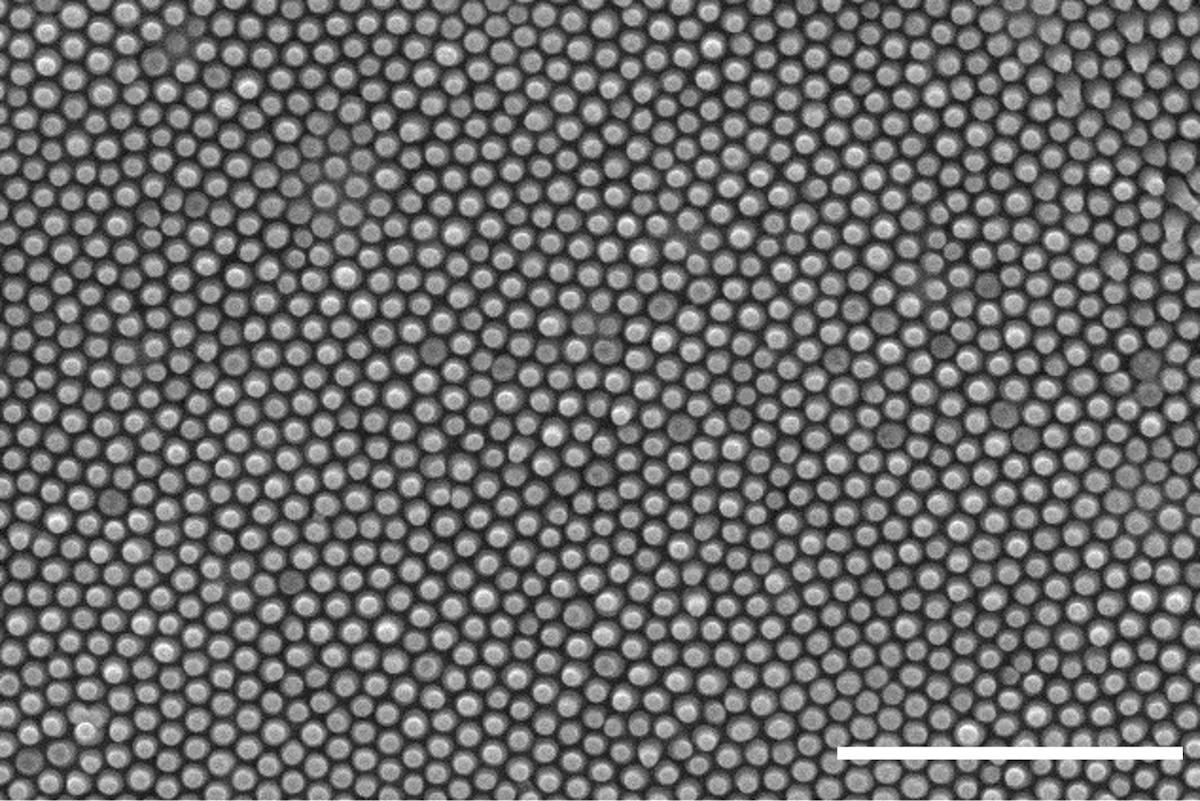 Scanning electron microscope image of replicated copper nanopillars. Scale bar is 2 micrometers. Credit: Yutao Chen.
This replication method can be paired with pulse electroplating, a metal deposition technique, to create copper nanopillar replicates. Chen studies them for applications like air and water filtration or to develop more conductive electrodes.
Scanning electron microscope image of replicated copper nanopillars. Scale bar is 2 micrometers. Credit: Yutao Chen.
This replication method can be paired with pulse electroplating, a metal deposition technique, to create copper nanopillar replicates. Chen studies them for applications like air and water filtration or to develop more conductive electrodes.
Chen uses Beckman’s environmental scanning electron microscope to observe Pseudomonas aeruginosa bacteria on natural and replicated nanopillars, and a confocal laser scanning microscope to evaluate how well biological and engineered nanopillar surfaces can destroy bacteria. In most cases, the nanopillars simply puncture or tear the outer membrane of the bacteria to repel or destroy it.
Chen’s images show that the nanopillars bend when engaging with bacteria.
It’s possible that the pillars are storing and releasing elastic energy when in contact with the bacteria which would ultimately stretch and tear the membrane, Chen said.
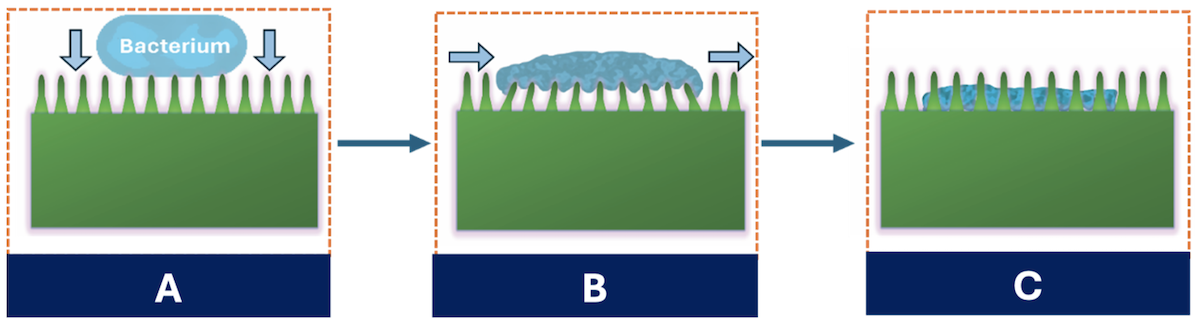 Bacteria comes into contact with nanopillars (A), which causes the nanopillars to bend and store elastic energy (B). Bacteria that attempt to move become damaged, stored elastic energy is released and the pillars return to their original position
(C). Credit: Yutao Chen.
Bacteria comes into contact with nanopillars (A), which causes the nanopillars to bend and store elastic energy (B). Bacteria that attempt to move become damaged, stored elastic energy is released and the pillars return to their original position
(C). Credit: Yutao Chen.
Using the scanning electron microscope, it can be hard to visualize the membrane at the exact moment when it becomes punctured because fluids begin to leak from the bacteria cell and obstruct the view. To determine which bacteria have been punctured, Chen uses the confocal laser microscope and a special dye that stains the bacteria — living bacteria cells with intact membranes will stain green while nonviable cells will stain red.
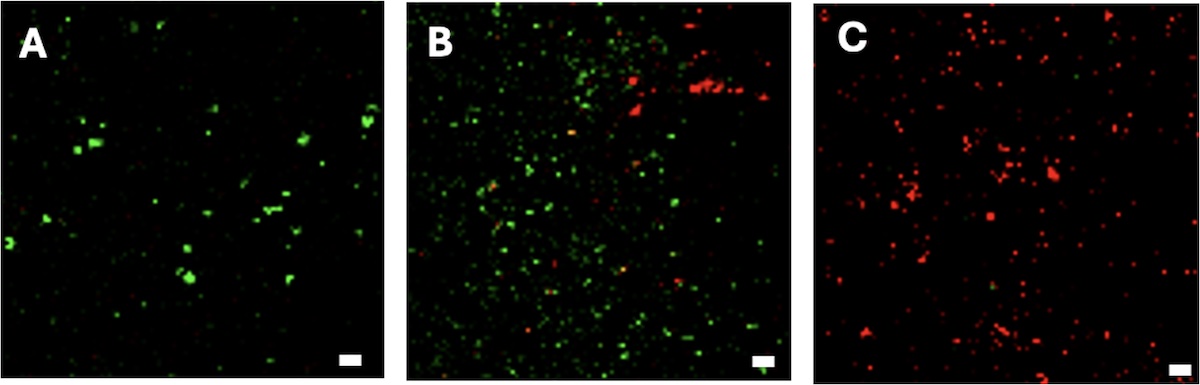 Confocal laser scanning microscope images of stain P. aeruginosa bacteria on various surfaces from least bactericidal (left) to most bactericidal (right). Smooth, untextured polystyrene (A), textured polystyrene with small nanopillars (B), textured
polystyrene with large nanopillars, replicated to scale. Green color indicates live bacteria; red color indicates dead bacteria. Scale bar is 5 micrometers. Credit: Yutao Chen.
Confocal laser scanning microscope images of stain P. aeruginosa bacteria on various surfaces from least bactericidal (left) to most bactericidal (right). Smooth, untextured polystyrene (A), textured polystyrene with small nanopillars (B), textured
polystyrene with large nanopillars, replicated to scale. Green color indicates live bacteria; red color indicates dead bacteria. Scale bar is 5 micrometers. Credit: Yutao Chen.
The size and structure of Chen’s replicated nanopillars closely match those of the natural nanopillars on cicada wings. By preserving the original dimensions and scale, Chen also preserves the functionality. The engineered nanopillars can destroy more than 95% of the bacteria within 3 hours.
There’s still a lot of work to do, Chen said.
Future plans include experimenting with different fabrication techniques and observing more dynamic interactions between bacteria and the replicated surfaces using microfluidics techniques. The microfluidics project involves using tiny channels that will allow Chen to flow liquid mixtures of bacteria across different nanopillar surfaces.
Editor's note:
Elizabeth Bello is a member of the ABCLab.
Media contact: Jenna Kurtzweil, kurtzwe2@illinois.edu
Beckman Institute for Advanced Science and Technology