Article
Recent research by Rafael C. Bernardi at the University of Illinois, Urbana-Champaign examines why a common tool in biotechnology — the binding of streptavidin to biotin — shows different mechanical resilience in different research labs.
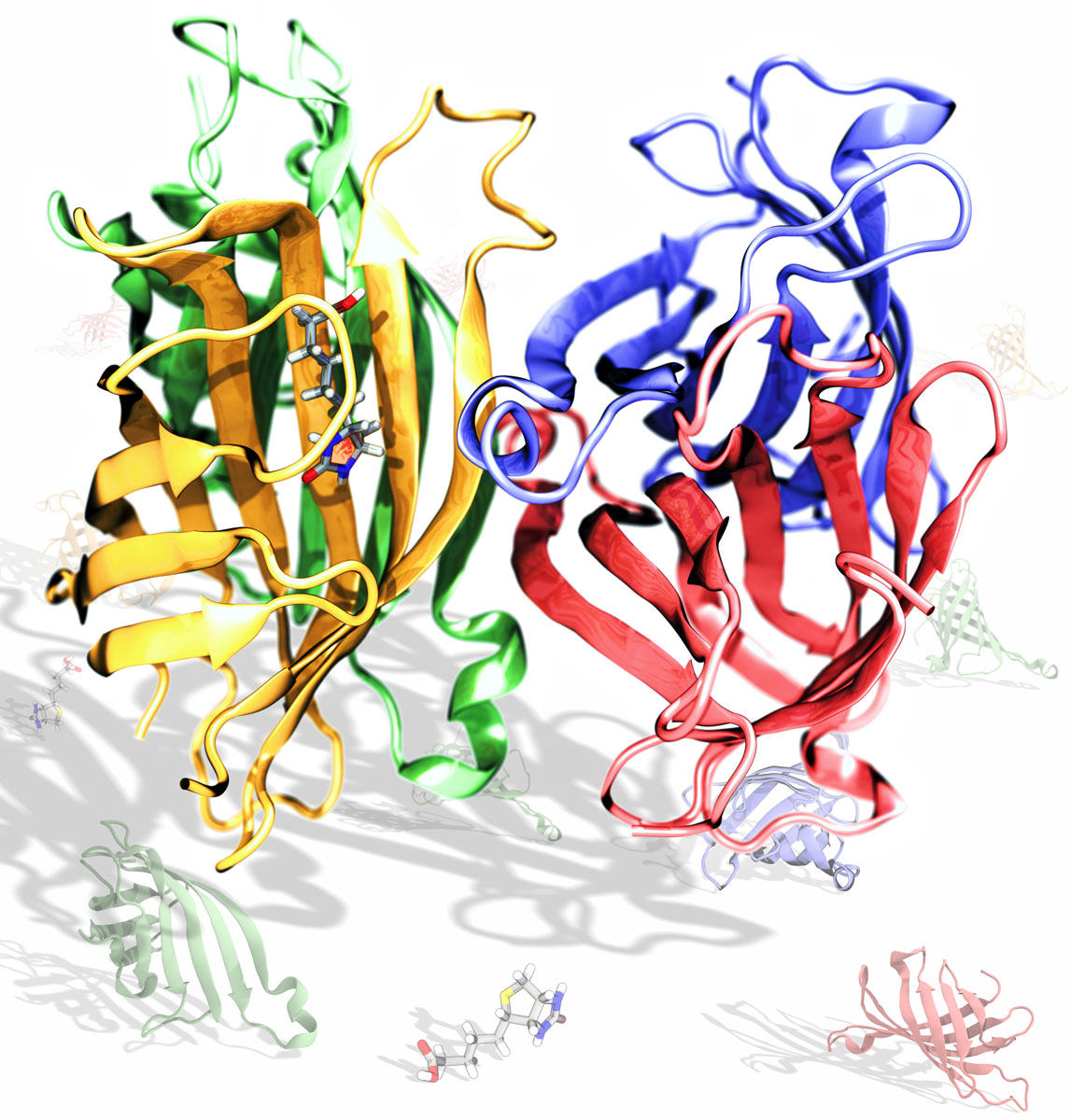 This rendering shows the tetrameric structure of streptavidin with a biotin molecule in one of the monomers. The art was prepared using VMD, by Rafael C. Bernardi.
This rendering shows the tetrameric structure of streptavidin with a biotin molecule in one of the monomers. The art was prepared using VMD, by Rafael C. Bernardi.
“This interaction has been a key tool in nearly 15,000 published manuscripts,” said Bernardi, a research scientist at the Beckman Institute for Advanced Science and Technology. “If you don’t do it in a specific way, your results might be inconclusive. It is like creating a building – you can use the same material to make something stable or unstable.”
The principle of using the binding of two molecules as a research tool is not limited to streptavidin/biotin systems. “This analysis can also be used in other experiments such as therapeutics and cancer treatments where, depending on the position
of the antibody, the stability of a complex is affected,” Bernardi said. “The technique is also being used in the work that is being done on the new SARS-CoV-2 coronavirus. The technique we are improving is a very common tool used by the
labs that are trying to understand the molecular basis of COVID-19.”
Bernardi conducted the research in collaboration with Hermann Gaub, a professor at Ludwig Maximilian University of Munich.The paper “Streptavidin/Biotin: Tethering Geometry Defines Unbinding Mechanics” was published in Science Advances.
Researchers have been using the interaction between streptavidin and biotin for the last 25 years to study, for instance, the folding and unfolding of proteins. In single-molecule folding studies, the protein is usually attached to a surface on one end and to biotin on the other. The streptavidin is then attached to the tip of an atomic force microscope, working as a handle to pull on the protein through the streptavidin/biotin interaction. The researchers measure this effect to understand how the protein folds and unfolds.
“Over the years we have noticed that the numbers associated with the strength of the interaction between streptavidin and biotin varies in different research papers,” Bernardi said. “If you’re measuring just this interaction, why aren’t the numbers always the same? Researchers didn’t think about it too much because, for most of them, biotinylation is just a tool.”
The researchers used computational analysis to show that the several streptavidin’s reactive groups, called reactive amines, can influence how streptavidin and biotin interact under mechanical stress. “Even with the controlled environments we used in our experiments, we got completely different results. You’re forcing biotin to take on a different path depending on where the streptavidin attachment takes place,” Bernardi said.
The biggest challenge the researchers faced was doing the computational analysis. “We need a lot of computer power to get these results. We were only able to do this because of Blue Waters,” Bernardi said, referring to the National Center for Supercomputing Applications’ supercomputer on the Illinois campus. To analyze the results, Bernardi employed NAMD, a widely used software for Molecular Dynamics, which was developed by the Theoretical and Computational Biophysics Group at the Beckman Institute.
The paper “Streptavidin/Biotin: Tethering Geometry Defines Unbinding Mechanics” can be found online at https://doi.org/10.1126/sciadv.aay5999.
Beckman Institute for Advanced Science and Technology