“Morphogenic Patterning in Synthetic Polymers”
Evan Lloyd, Beckman Institute Graduate Fellow, Ph.D. student in chemical and biomolecular engineering
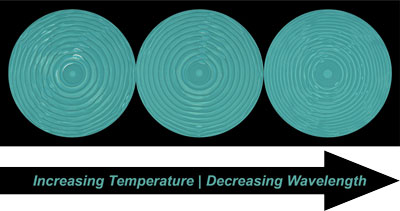
Complexity in biological systems spontaneously emerges from an initial state of symmetry through coupled reaction and diffusion, a process known as morphogenesis. In this work, we explore the coupled reaction and thermal diffusion inherent to frontal ring-opening metathesis polymerization (FROMP) of dicyclopentadiene (DCPD) as a synthetic mimic to biological morphogenesis. Propagation instabilities arise from an initial state of symmetry and generate patterns of thermal fluctuations on multiple length scales. Incorporation of thermo-active small molecules enables spontaneous patterning of the optical, chemical, and mechanical properties of structural thermosets. Material stiffness and glass transition temperatures were found to vary by up to two-fold and 20°C, respectively. Control over patterns by tuning reaction and diffusion rates will also be discussed.
“Non-targeted D-amino Acid Containing Neuropeptide Discovery in Animals”
David Mast, Ph.D. student in chemistry
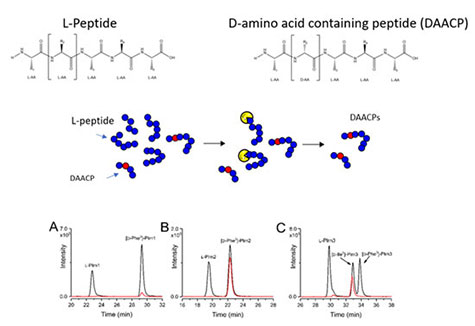
D-amino acid-containing peptides result from an unusual post translational modification (PTM) where an all-L peptide is isomerized at a single amino acid residue. The biological relevance of DAACPs is not well understood because the prevalence of isomerization among animal neuropeptides is largely unknown. DAACPs are particularly difficult to identify and measure due to challenges differentiating DAACPs from all-L peptides in complex biological extracts using modern mass spectrometry-based approaches. Furthermore, stereochemistry is not routinely evaluated in “peptidomics” experiments. Here, non-targeted stereoselective enzymatic screening, liquid chromatography tandem mass spectrometry and trapped ion mobility spectrometry were used to identify novel DAACPs in the central nervous system of the Aplysia californica.
“Modeling a Ternary Coagulation Complex on a Lipid Bilayer Using Molecular Dynamics Solutions”
Melanie Muller, M.D. and Ph.D. student in biophysics and quantitative biology
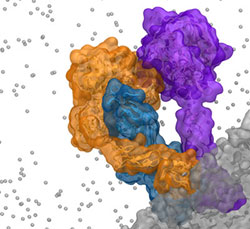
Extrinsic complex formation on the surface of activated platelets is a critical step in the activation of the coagulation cascade. Lipid dependency of complex formation, as well as flexibility of the component proteins, has made atomic-level experimental characterization of the complex structure prohibitively difficult. Using a novel methodology combining rigid-body docking and extensive non-equilibrium molecular dynamics, we have developed the first atomic-level model of the extrinsic complex to include membrane interactions. Molecular dynamics simulations were used to drive complex formation on the surface of an anionic phospholipid bilayer in independent simulations of 550 ns in length. A specialized membrane model, the highly mobile membrane mimetic, was employed to allow increased sampling of protein-lipid interactions during this deliberate complex formation. Putative key protein-protein and protein-lipid interactions involved in complex formation were identified based on final complex structures and are consistent with previous experimental mutagenesis studies.