“High-speed Fluorescence Lifetime Imaging Microscopy for Label-free Metabolic Imaging of Dynamic Processes in Living Cells”
Andrew J. Bower, Ph.D. student in electrical and computer engineering, Beckman Institute Graduate Fellow, Bioimaging Science and Technology Group
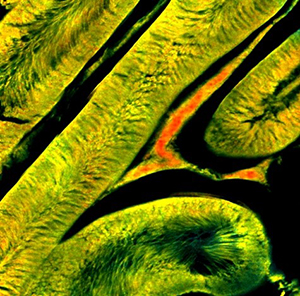
Two-photon fluorescence lifetime imaging microscopy (TP-FLIM) of the metabolic coenzyme NADH has been used to investigate metabolic perturbations in living cells and tissues in a label-free manner. While the instruments traditionally utilized are incredibly sensitive to the local molecular changes associated with these metabolic processes, they are inherently slow due to the detection technique utilized, prohibiting study of metabolic dynamics. Here, a video-rate TP-FLIM system was developed based on a direct fluorescence decay measurement technique allowing the study of highly dynamic metabolic processes. Further, this high-speed imaging system was used to characterize the dynamic process of apoptosis in vitro with high temporal resolution, previously not possible using standard FLIM instruments. Results show a significant increase in lifetime within 10 seconds of the addition of high concentrations of the apoptosis-inducing drug staurosporine that remains elevated at 15 minutes, agreeing with previous results. The dose-dependent response at these critical early time points will be further shown to fully characterize this process and potentially identify drug-resistance within large cell populations. This high-throughput imaging system is capable of high-speed, deep-tissue imaging at cellular resolution, extending the capabilities of these metabolic imaging approaches and potentially enabling study of a wide variety of cellular processes.
“Modularity of the Microcircuitry of the Lateral Cortex of the Mouse Inferior Colliculus”
Alexandria Marie Lesicko, Ph.D. student in neuroscience, Cellular and Molecular Foundations of Intelligent Behavior Group
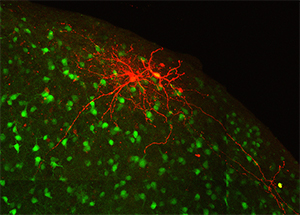
The inferior colliculus, or auditory midbrain, lies at the center of the auditory pathway and serves as an obligatory integration hub for all sound information passing from the cochlea to the auditory cortex. The outer shell of the inferior colliculus, known as the lateral cortex, is unique in that, in addition to auditory information, it receives heavy visual and somatosensory input and is thought to play a role in multisensory integration. Furthermore, this structure contains discrete patches, or modules, filled with high concentrations of neurochemicals that are known to regulate plasticity, metabolic activity, and inhibition. Recent data from our laboratory have shown that, despite its multimodal functions, the auditory and somatosensory inputs to the lateral cortex are segregated and correlated with the distribution of neurochemical modules. This talk will highlight findings regarding the intrinsic and extrinsic connectivity of the lateral cortex, and how its unique modular organization may give rise to partially segregated processing streams for auditory and multisensory information.
“Activatable DNA Aptamer Probes for Selective In Vivo Photoacoustic Imaging of a Wide Range of Targets”
Nitya Sai Reddy Satyavolu, Ph.D. student in chemistry, Beckman Institute Graduate Fellow, 3D Micro- and Nanosystems Group
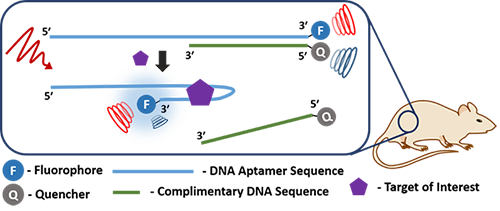
Photoacoustic Imaging (PAI) as an imaging modality is unique due to its ability of scaling spatial resolution and imaging depth across both optical and ultrasonic dimensions. In principle, any molecule or material that has the ability to absorb around a specific wavelength of light has the ability to convert the absorbed light to acoustic waves when excited at that particular wavelength using a pulsed laser. However, there are shortcomings that most known contrast agents have, such as the lack of selectivity to a specific target. While recent literature report some selective contrast agents, the way most agents have been generated is not general. Moreover, rationally designing and synthesizing target specific molecular contrast agents can be an arduous task. Adopting functional DNA (i.e., DNAzymes or aptamers tagged with PAI contrast agents) impart specificity for a plethora of targets ranging from proteins to small molecular metabolites such as ATP or metal ions. Additionally, functional DNA can be easily integrated with nanomaterials through simple conjugation chemistries. We demonstrate that DNA nanotechnology can be utilized in conjunction with PAI for the ratiometric detection of proteins such as PDGF-BB and thrombin making the PAI platform more viable in a biomedical setting.